| Glutamate receptor anatgonists from the spider Argiope lobata venom | E. V. Grishin, T. M. Volkova, A. S. Arseniev, Biorg. Khim. 1988, 14, 883-892 | | | | |
| Isolation and structure analysis of components from venom of the spider Argiope lobata | E. V. Grishin, T. M. Volkova, A. S. Arseniev, Toxicon 1989, 27, 541-549 | A. lobata | Pseudoargiopinin 3 | FAB-MS, NMR (ns), Amino acid analysis, Activity-studies | Link |
| Argiopine, argiopinines, and pseudoargiopinines as glutamate receptor blockers in hippocampal neurons | N. I. Kiskin, O. A. Kryshtal, A. Ya. Tsyndrenko, T. M. Volkova, E. V. Grishin, Neurophysiology 1990, 21, 525-532 | A. lobata | Pseudoargiopinin 3 | Activity-studies | Link |
| Antropod toxins as leads for novel insecticides: An assessment of polyamine amides as glutamate antagonists | I. S. Blagbrough, P. T. H. Brackley, M. Bruce, B. W. Bycroft, A. J. Mather, S. Millington, H. L. Sudan, P. N. R. Usherwood, Toxicon 1992, 30, 303-322 | | Pseudoargiopinin 3 | Activity-studies | Link |
| Polyamine toxins from spiders and wasps | A. Schäfer, H. Benz, W. Fiedler, A. Guggisberg, S. Bienz, M. Hesse, The Alkaloids 1994, 45, 1-125 | A. lobata | Arg 373 | Review | Link |
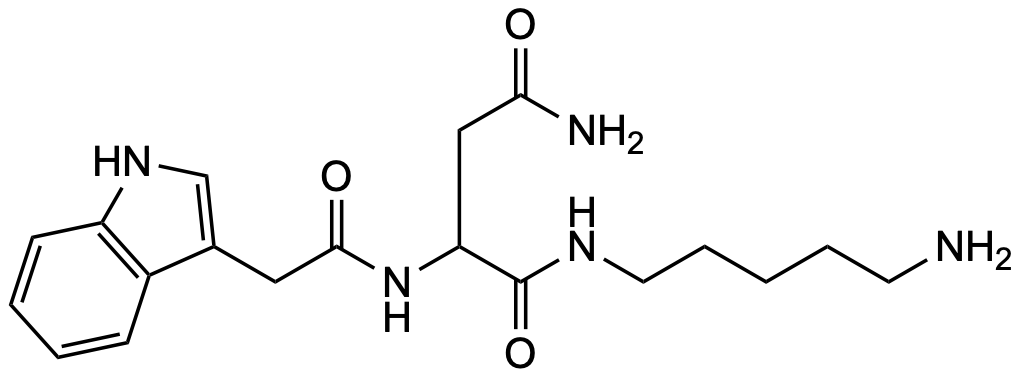
| Solid-phase synthesis of peptide aminoalkylamides using an allyl linker | K. Kaljuste, A. Unden, Tetrahedron Letters 1996, 37, 3031-3034 | — | Pseudioargiopinin 3 (7) | Synthesis, NMR | Link |
| Versatile Dde-based primary amine linkers for solid phase synthesis | S. R. Chhabra, A. N. Khan, B. W. Bycroft, Tetrahedron Letters 1998, 39, 3585-3588 | — | Pseudoargiopinin 3 (7) | Synthesis, NMR (ns) | Link |
| Total synthesis of NPTX-643, a neurotoxin of the Madagascar spider (Nephilengys borbonica) having a novel acylpolyamine structure | M. Miyashita, T. Kanemura, M. Matsushita, S. Hatakeyama, Y. Itagaki, T. Nakajima, M. Miyazawa, H. Irie, Heterocycles 1998, 47, 171-175 | — | | Synthesis | Link |
| Acylpolyamines: Mass spectrometric analytical methods for Araneidae spider acylpolyamines | Y. Itagaki , T. Nakajima , Toxin Rev. 2000, 19, 23-52 | A. lobata | Arg 373 | Review | Link |
| Elucidation of the structure and synthesis of neuroprotective low molecular mass components of the Parawixia bistriata spider venom | Y. M. Forster, J. L. Green, A. Khatiwada, J. L. Liberato, P. A. Narayana Reddy, J. M. Salvino, S. Bienz, L. Bigler, W. Ferreira dos Santos, A. C. K. Fontana, ACS Chem Neurosci., 2020 | P. bistriata | | ESI-MS/MS | Link |