IndAc343
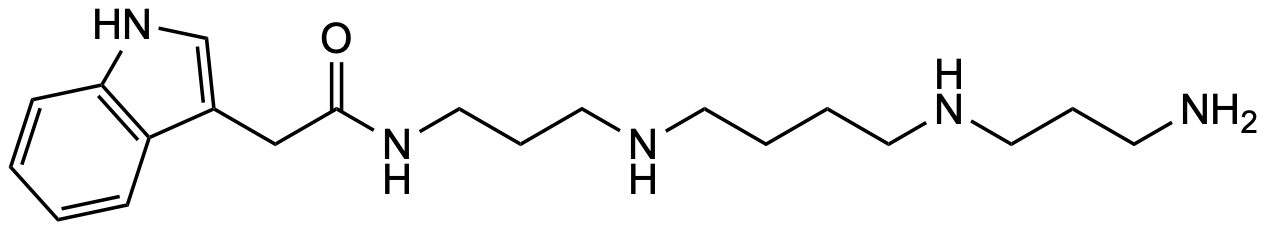
General Description
| Name | Value |
|---|---|
| Level | S-3 / C-1 |
| Discovered | 2009 / O. lugubris |
| Synonym | HO 359b / OZ 359 |
| Molecular formula | C₂₀H₃₃N₅O |
| CAS | 122306-13-2 |
| SMILES | O=C(NCCCNCCCCNCCCN)CC1=CNC2=C1C=CC=C2 |
| InChI | InChI=1S/C20H33N5O/c21-9-5-12-22-10-3-4-11-23-13-6-14-24-20(26)15-17-16-25-19-8-2-1-7-18(17)19/h1-2,7-8,16,22-23,25H,3-6,9-15,21H2,(H,24,26) |
| Precursor 1 [M+H]⁺ | 360.27579 |
| Precursor 2 [M+2H]²⁺ | 180.64153 |
| Precursor 3 | |
| HDX | 6 |
| Precursor HDX 1 [M(D₆)+D]⁺ | 367.31972 |
| Precursor HDX 2 [M(D₆)+2D]²⁺ | 184.66664 |
| Precursor HDX 3 | |
| Rt | 7.84 |
| Rt HDX | 6.45 |
Calculated MS/MS fragments
| # | a | b | c | ta | z | y | tz |
|---|---|---|---|---|---|---|---|
| 1 | 215.11789 | 197.10732 | 198.09134 | 232.14444 | 58.06513 | 41.03858 | 75.09167 |
| 2 | 286.19139 | 268.18082 | 269.16484 | 303.21794 | 129.13862 | 112.11208 | 146.16517 |
| 3 | 343.24924 | 325.23867 | 326.22269 | 360.27579 | 186.19647 | 169.16993 | 203.22302 |
Additional MS/MS fragments
| m/z | Annotation |
|---|---|
| 130.06513 | a’ |
| 158.06004 | a0 |
Recorded MS/MS spectra
| Precursor | Co-eluting | Spider | Source | Author | |
|---|---|---|---|---|---|
| Data | 360.27579 | IndAc334 | A. aperta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 360.27579 | IndAc334 | A. aperta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | IndAc334 | A. aperta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 360.27579 | IndAc334 | A. potteri | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | IndAc334 | A. potteri | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 360.27579 | H. curta | Fauna Laboratories Ltd., KAZ | Y. M. Forster | |
| Data | HDX | IndAc334 | H. curta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 360.27579 | IndAc334 | Hololena sp. | Spider Pharm, USA | Y. M. Forster |
| Data | 180.64153 | IndAc334 | Hololena sp. | Spider Pharm, USA | Y. M. Forster |
| Data | HDX | IndAc334 | Hololena sp. | Spider Pharm, USA | Y. M. Forster |
| Data | 360.27579 | IndAc334 | P. luctuosa | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | IndAc334 | P. luctuosa | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
References
| Title | Reference | Spider | Name | Content | Link |
|---|---|---|---|---|---|
| Acylpolyamines mimic the action of Joro spider toxin (JSTX) on crustacean muscle glutamate receptors | T. Asami, H. Kagechika, Y. Hashimoto, K. Shudo, A. Miwa, N. Kawai, T. Nakajima, Biomedical Research 1989, 10, 185-189 | — | Synthesis, Activity-studies | Link | |
| Characterization and synthesis of a new calcium antagonist from the venom of a Fishing spider | K. D. McCormick, K. Kobayashi, S. M. Goldin, N. Laxma Reddy, J. Meinwald, Tetrahedron 1993, 49, 11155 | — | (1) | Synthesis, NMR, FAB-MS/MS | Link |
| Amino acid/spermine conjugates: Polyamine amides as potent spermidine uptake inhibitors | M. R. Burns, C. L. Carlson, S. M. Vanderwerf, J. R. Ziemer, R. S. Weeks, F. Cai, H. K. Webb, G. F. Graminski, J. Med. Chem. 2001, 44, 3632-3644 | — | (13) | Synthesis, NMR, Activity-studies | Link |
| Versatile procedure for asymmetric and orthogonal protection of symmetric polyamines and its advantages for solid phase synthesis | F. Hahn, U. Schepers, J. Comb. Chem. 2008, 10, 267-273 | — | HO 359b (10) | Synthesis, NMR | Link |
| Development of a high-resolution MS-based method for the structural elucidation of polyamine spider toxins | S. Eichenberger, PhD-Thesis, University of Zurich 2009, 1-156 | O. lugubris | OZ 359 | nLC-ESI-MS/MS | Link |
| Low molecular mass compounds in spider venom | Y. M. Forster, S. Bienz, L. Bigler, 2020, in preparation | div. | Link |
Spider species
| Spider species | Family | Discovered |
|---|---|---|
| Agelenopsis aperta | Agelenidae | 2020 / Y. M. Forster |
| Agelenopsis potteri | Agelenidae | 2020 / Y. M. Forster |
| Hololena curta | Agelenidae | 2020 / Y. M. Forster |
| Hololena sp. | Agelenidae | 2020 / Y. M. Forster |
| Ozyptila lugubris | Thomisidae | 2009 / S. Eichenberger |
| Pireneitega luctuosa | Agelenidae | 2020 / Y. M. Forster |