Navigation :
IndAc3334
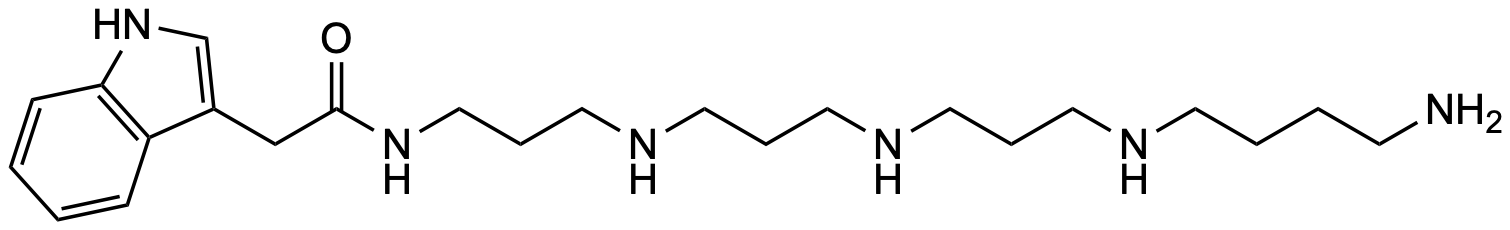
General Description
| Name | Value |
|---|
| Level | S-1 / C-1 |
| Discovered | 2001 / A. aperta |
| Synonym | AG 416a |
| Molecular formula | C₂₃H₄₀N₆O |
| CAS | 389872-68-8 |
| SMILES | O=C(NCCCNCCCNCCCNCCCCN)CC1=CNC2=C1C=CC=C2 |
| InChI | InChI=1S/C23H40N6O/c24-10-3-4-11-25-12-5-13-26-14-6-15-27-16-7-17-28-23(30)18-20-19-29-22-9-2-1-8-21(20)22/h1-2,8-9,19,25-27,29H,3-7,10-18,24H2,(H,28,30) |
| |
| Precursor 1 [M+H]⁺ | 417.33364 |
| Precursor 2 [M+2H]²⁺ | 209.17046 |
| Precursor 3 | |
| |
| HDX | 7 |
| Precursor HDX 1 [M(D₇)+D]⁺ | 425.38385 |
| Precursor HDX 2 [M(D₇)+2D]²⁺ | 213.69870 |
| Precursor HDX 3 | |
| |
| Rt | 7.59 |
| Rt HDX | 6.21 |
Calculated MS/MS fragments
| # | a | b | c | ta | z | y | tz |
|---|
| 1 | 215.11789 | 197.10732 | 198.09134 | 232.14444 | 72.08078 | 55.05423 | 89.10732 |
| 2 | 272.17574 | 254.16517 | 255.14919 | 289.20229 | 129.13862 | 112.11208 | 146.16517 |
| 3 | 329.23359 | 311.22302 | 312.20704 | 346.26014 | 186.19647 | 169.16993 | 203.22302 |
| 4 | 400.30709 | 382.29652 | 383.28054 | 417.33364 | 243.25432 | 226.22777 | 260.28087 |
Additional MS/MS fragments
| m/z | Annotation |
|---|
| 130.06513 | a’ |
| 158.06004 | a0 |
Recorded MS/MS spectra
| pdf | Precursor | Co-eluting | Spider | Source | Author |
|---|
| Data | 417.33364 | IndAc3343 | A. aperta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 209.17046 | IndAc3343 | A. aperta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | IndAc3343 / IndAc3433 | A. aperta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 417.33364 | IndAc3343 | A. potteri | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 209.17046 | IndAc3343 | A. potteri | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | IndAc3343 | A. potteri | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 417.33364 | | E. agrestis | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | | E. agrestis | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 417.33364 | | H. curta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 209.17046 | IndAc3343 / IndAc3433 | H. curta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | IndAc3343 | H. curta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 417.33364 | | Hololena sp. | Spider Pharm, USA | Y. M. Forster |
| Data | 209.17046 | IndAc3343 | Hololena sp. | Spider Pharm, USA | Y. M. Forster |
| Data | HDX | IndAc3343 | Hololena sp. | Spider Pharm, USA | Y. M. Forster |
| Data | 417.33364 | | P. luctuosa | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | | P. luctuosa | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
References
| Title | Reference | spider | name | content | link |
|---|
| The acylpolyamines from the venom of the spider Agelenopsis aperta | S. Chesnov, L. Bigler, M. Hesse, Helv. Chim. Acta 2001, 84, 2178-2197 | A. aperta | AG 416a | APCI-MS/MS | Link |
| Solid-phase synthesis of polyamine spider toxins and correlation with natural products by HPLC-MS/MS | N. Manov, M. Tzouros, S. Chesnov, L. Bigler, S. Bienz, Helv. Chim. Acta 2002, 85, 2827-2846 | A. aperta | | Synthesis, NMR, APCI-MS/MS | Link |
| Tandem mass spectrometric investigation of acylpolyamines of spider venoms and their 15N-labeled derivatives | M. Tzouros, N. Manov, S. Bienz, L. Bigler, J. Am. Soc. Mass Spectrom. 2004, 15, 1636-1643 | | | APCI-MS/MS | Link |
| A template approach for the characterization of linear polyamines and derivatives in spider venom | M. Tzouros, S. Chesnov, L. Bigler, S. Bienz, Eur. J. Mass Spectrom. 2013, 19, 1, 57-69 | A. aperta | AG 416a | APCI-MS/MS | Link |
| Low molecular mass compounds in spider venom | Y. M. Forster, S. Bienz, L. Bigler, 2020, in preparation | div. | | | Link |
Spider species
| Spider species | Family | Discovered |
|---|
| Agelenopsis aperta | Agelenidae | 2001 / S. Chesnov |
| Agelenopsis potteri | Agelenidae | 2020 / Y. M. Forster |
| Eratigena agrestis | Agelenidae | 2020 / Y. M. Forster |
| Hololena curta | Agelenidae | 2020 / Y. M. Forster |
| Hololena sp. | Agelenidae | 2020 / Y. M. Forster |
| Pireneitega luctuosa | Agelenidae | 2020 / Y. M. Forster |
