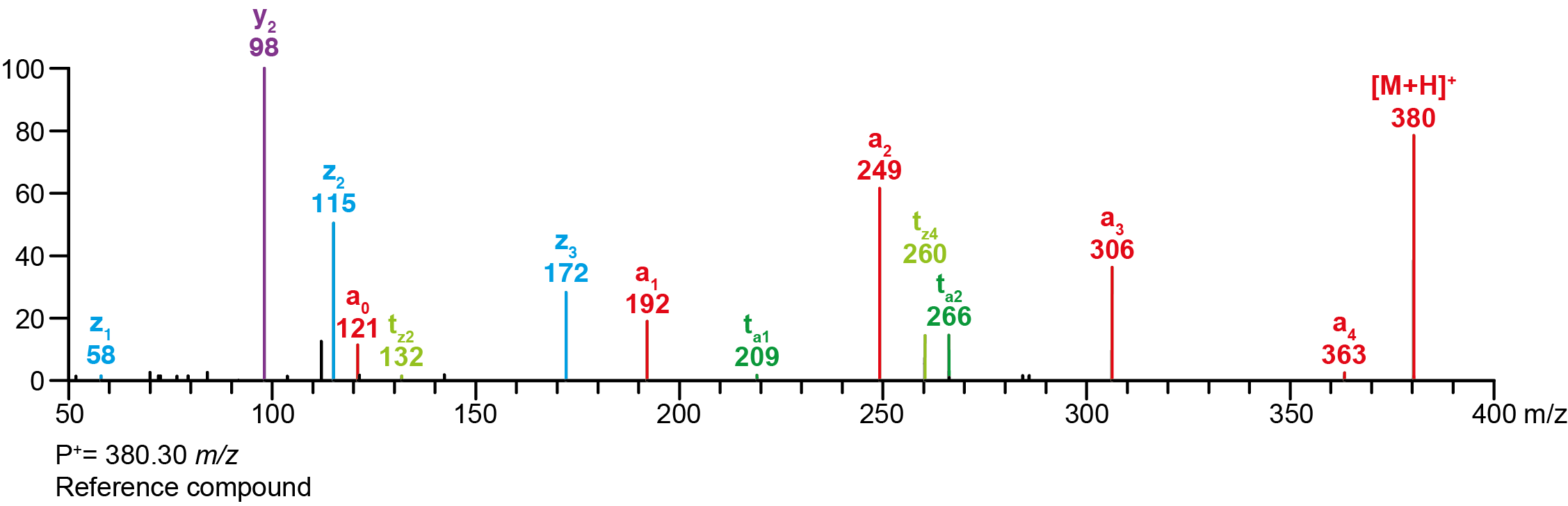Navigation :
4-OH-Bz4333
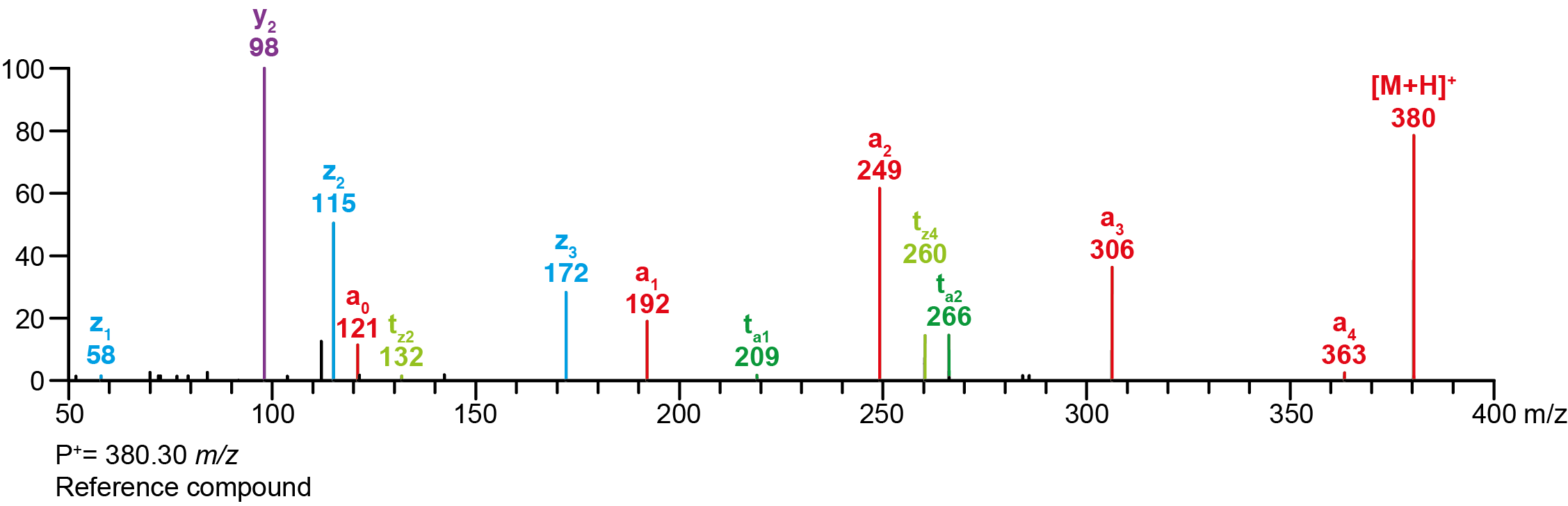
General Description
| Name | Value |
|---|
| Level | S-1 / C-1 |
| Discovered | 2001 / A. aperta |
| Synonym | AG 379a |
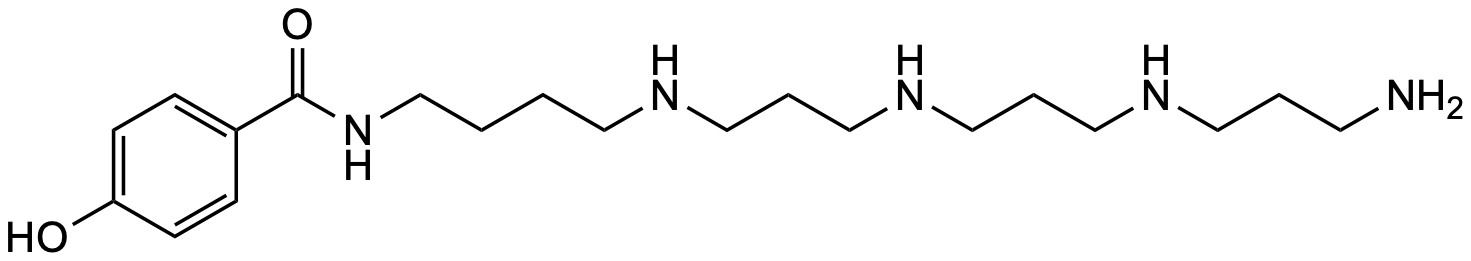
| Molecular formula | C₂₀H₃₇N₅O₂ |
| CAS | 389872-50-8 |
| SMILES | O=C(NCCCCNCCCNCCCNCCCN)C1=CC=C(O)C=C1 |
| InChI | InChI=1S/C20H37N5O2/c21-10-3-12-23-14-5-16-24-15-4-13-22-11-1-2-17-25-20(27)18-6-8-19(26)9-7-18/h6-9,22-24,26H,1-5,10-17,21H2,(H,25,27) |
| |
| Precursor 1 [M+H]⁺ | 380.30200 |
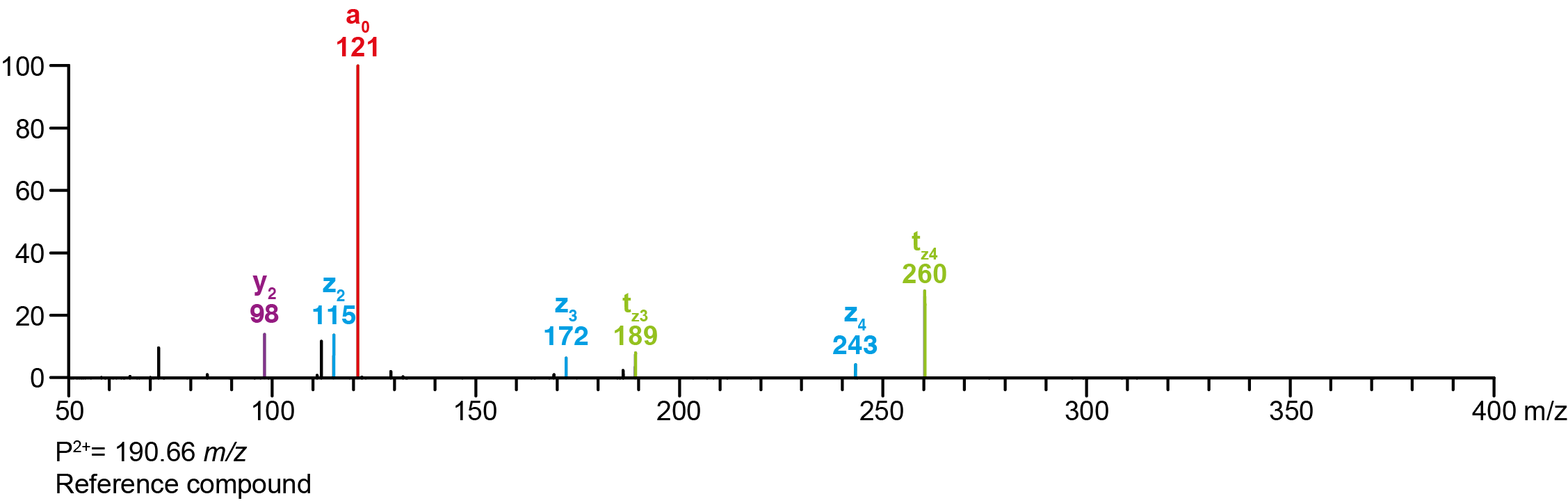
| Precursor 2 [M+2H]²⁺ | 190.65464 |
| Precursor 3 | |
| |
| HDX | 7 |
| Precursor HDX 1 [M(D₇)+D]⁺ | 388.35222 |
| Precursor HDX 2 [M(D₇)+2D]²⁺ | 195.18288 |
| Precursor HDX 3 | |
| |
| Rt | 3.87 |
| Rt HDX | 2.87 |
Calculated MS/MS fragments
| # | a | b | c | ta | z | y | tz |
|---|
| 1 | 192.10191 | 174.09134 | 175.07536 | 209.12845 | 58.06513 | 41.03858 | 75.09167 |
| 2 | 249.15975 | 231.14919 | 232.13321 | 266.18630 | 115.12297 | 98.09643 | 132.14952 |
| 3 | 306.21760 | 288.20704 | 289.19105 | 323.24415 | 172.18082 | 155.15428 | 189.20737 |
| 4 | 363.27545 | 345.26489 | 346.24890 | 380.30200 | 243.25432 | 226.22777 | 260.28087 |
Additional MS/MS fragments
| m/z | Annotation |
|---|
| 112.11208 | y2’ |
| 121.02841 | a0 |
| 362.29144 | bw |
Recorded MS/MS spectra
| pdf | Precursor | Co-eluting | Spider | Source | Author |
|---|
| Data | 380.30200 | | synth. 4-OH-Bz4333 | UZH Bienz lab, CHE | Y. M. Forster |
| Data | 190.65464 | | synth. 4-OH-Bz4333 | UZH Bienz lab, CHE | Y. M. Forster |
| Data | 380.30200 | | A. aperta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 190.65464 | | A. aperta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 380.30200 | | A. potteri | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 190.65464 | | A. potteri | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | | A. potteri | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 380.30200 | | E. agrestis | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 190.65464 | | E. agrestis | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | | E. agrestis | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 380.30200 | | H. curta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 190.65464 | | H. curta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | | H. curta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 380.30200 | | Hololena sp. | Spider Pharm, USA | Y. M. Forster |
| Data | 190.65464 | | Hololena sp. | Spider Pharm, USA | Y. M. Forster |
| Data | HDX | | Hololena sp. | Spider Pharm, USA | Y. M. Forster |
| Data | 380.30200 | | P. luctuosa | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | | P. luctuosa | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
References
| Title | Reference | Spider | Name | Content | Link |
|---|
| The acylpolyamines from the venom of the spider Agelenopsis aperta | S. Chesnov, L. Bigler, M. Hesse, Helv. Chim. Acta 2001, 84, 2178-2197 | A. aperta | AG 379a | APCI-MS/MS | Link |
| Solid-phase synthesis of polyamine spider toxins and correlation with natural products by HPLC-MS/MS | N. Manov, M. Tzouros, S. Chesnov, L. Bigler, S. Bienz, Helv. Chim. Acta 2002, 85, 2827-2846 | A. aperta | | Synthesis, NMR, APCI-MS/MS | Link |
| Low molecular mass compounds in spider venom | Y. M. Forster, S. Bienz, L. Bigler, 2020, in preparation | div. | | | Link |
Spider species
| Spider species | Family | Discovered |
|---|
| Agelenopsis aperta | Agelenidae | 2001 / S. Chesnov |
| Agelenopsis potteri | Agelenidae | 2020 / Y. M. Forster |
| Hololena curta | Agelenidae | 2020 / Y. M. Forster |
| Hololena sp. | Agelenidae | 2020 / Y. M. Forster |
| Pireneitega luctuosa | Agelenidae | 2020 / Y. M. Forster |