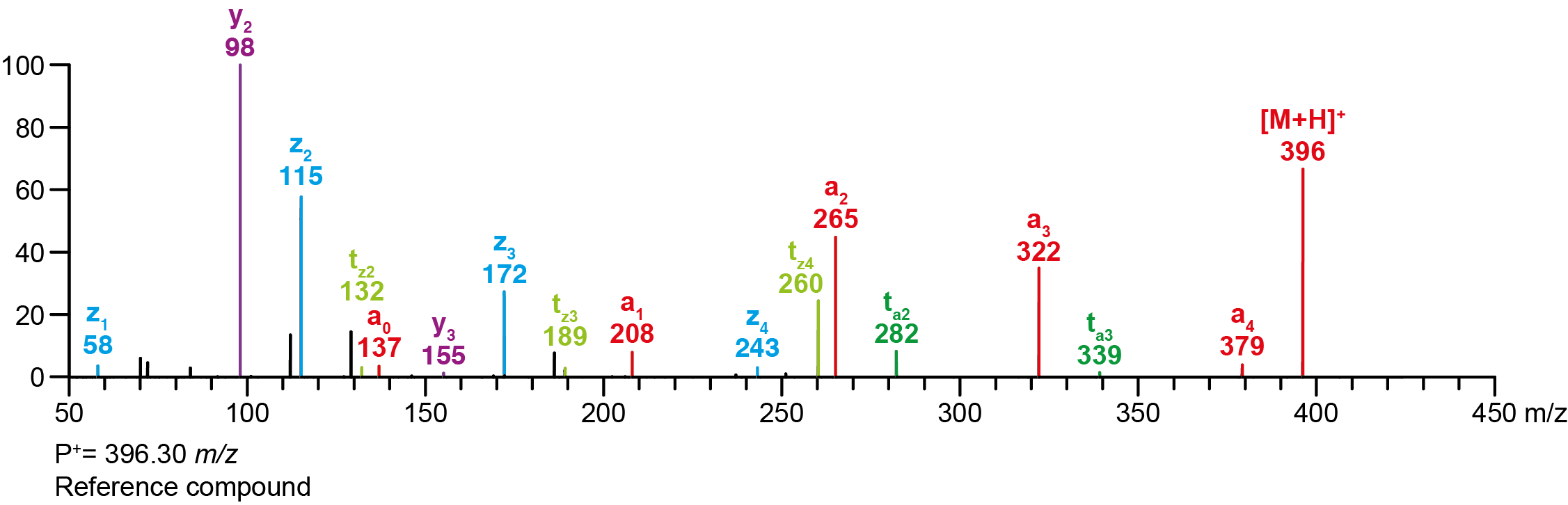Navigation :
2,5-(OH)₂-Bz4333
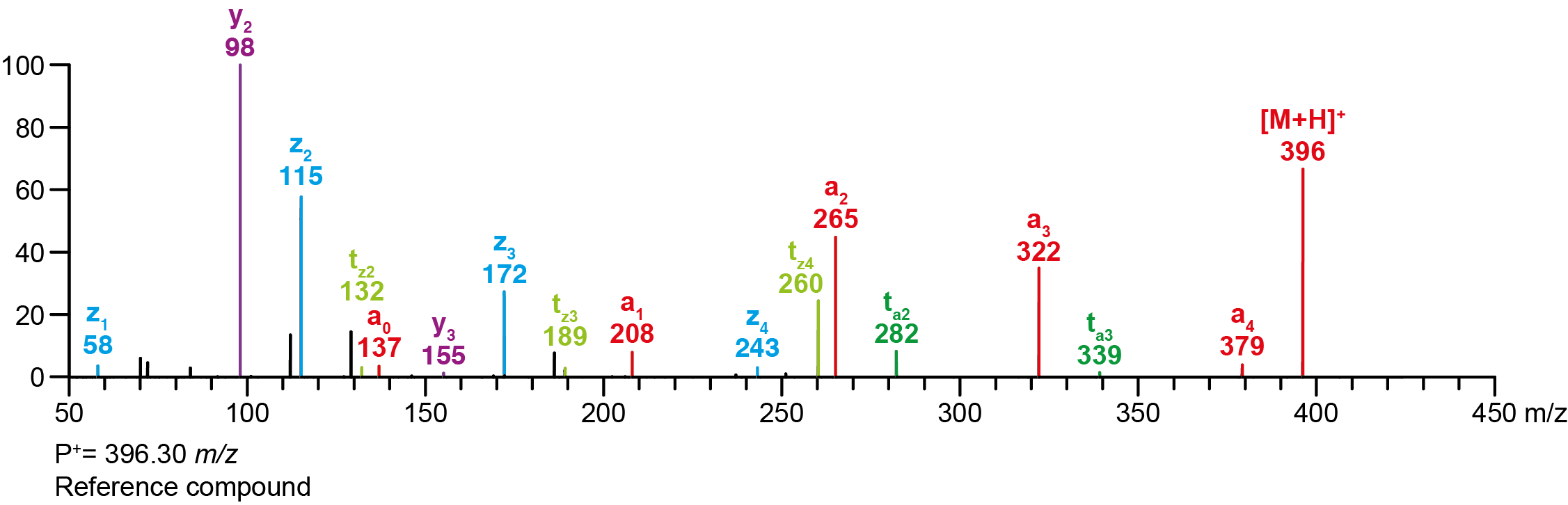
General Description
| Name | Value |
|---|
| Level | S-1 / C-1 |
| Discovered | 1991 / H. curta |
| Synonym | AG 395a / HO 395 |
| Molecular formula | C₂₀H₃₇N₅O₃ |
| CAS | 133823-89-9 |
| SMILES | O=C(NCCCCNCCCNCCCNCCCN)C1=C(O)C=CC(O)=C1 |
| InChI | InChI=1S/C20H37N5O3/c21-8-3-10-23-12-5-14-24-13-4-11-22-9-1-2-15-25-20(28)18-16-17(26)6-7-19(18)27/h6-7,16,22-24,26-27H,1-5,8-15,21H2,(H,25,28) |
| |
| Precursor 1 [M+H]⁺ | 396.29692 |
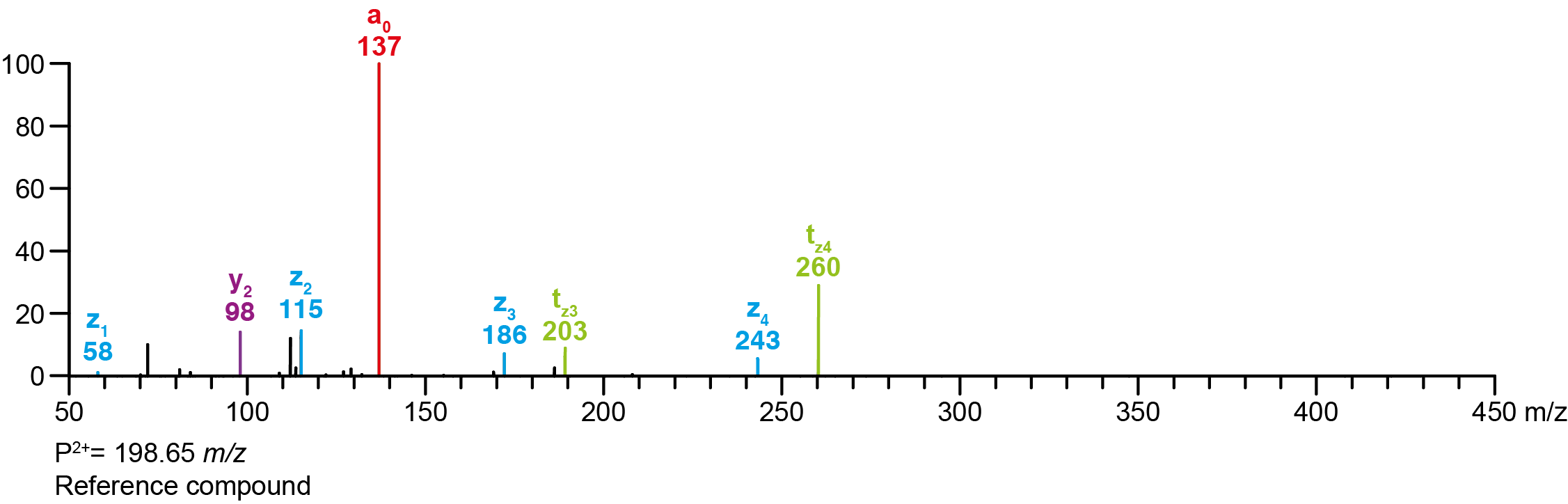
| Precursor 2 [M+2H]²⁺ | 198.65210 |
| Precursor 3 | |
| |
| HDX | 8 |
| Precursor HDX 1 [M(D₈)+D]⁺ | 405.35341 |
| Precursor HDX 2 [M(D₈)+2D]²⁺ | 203.68348 |
| Precursor HDX 3 | |
| |
| Rt | 6.21 |
| Rt HDX | 4.65 |
Calculated MS/MS fragments
| # | a | b | c | ta | z | y | tz |
|---|
| 1 | 208.09682 | 190.08626 | 191.07027 | 225.12337 | 58.06513 | 41.03858 | 75.09167 |
| 2 | 265.15467 | 247.14410 | 248.12812 | 282.18122 | 115.12297 | 98.09643 | 132.14952 |
| 3 | 322.21252 | 304.20195 | 305.18597 | 339.23907 | 172.18082 | 155.15428 | 189.20737 |
| 4 | 379.27037 | 361.25980 | 362.24382 | 396.29692 | 243.25432 | 226.22777 | 260.28087 |
Additional MS/MS fragments
| m/z | Annotation |
|---|
| 72.08078 | z1’ |
| 112.11208 | y2’ |
| 129.13862 | z2’ |
| 137.02332 | a0 |
Recorded MS/MS spectra
| pdf | Precursor | Co-eluting | Spider | Source | Author |
|---|
| Data | 396.29692 | | synth. 2,5-(OH)₂-Bz4333 | UZH Bienz lab, CHE | Y. M. Forster |
| Data | 198.65210 | | synth. 2,5-(OH)₂-Bz4333 | UZH Bienz lab, CHE | Y. M. Forster |
| Data | 396.29692 | | A. potteri | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | | A. potteri | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 396.29692 | | H. curta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 198.65210 | | H. curta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | HDX | | H. curta | Fauna Laboratories Ltd., KAZ | Y. M. Forster |
| Data | 396.29692 | | Hololena sp. | Spider Pharm, USA | Y. M. Forster |
| Data | 198.65210 | | Hololena sp. | Spider Pharm, USA | Y. M. Forster |
| Data | HDX | | Hololena sp. | Spider Pharm, USA | Y. M. Forster |
References
| Title | Reference | Spider | Name | Content | Link |
|---|
| Paralytic and insecticidal toxins from the funnel web spider, Hololena curta | G. B. Quistad, C. C. Reuter, W. S. Skinner, P. A. Dennis, S. Suwanrumpha, E. W. Fu, Toxicon 1991, 29, 329-336 | H. curta | HO 395 | FAB-MS/MS (ns), Activity-studies | Link |
| Polyamine toxins from spiders and wasps | A. Schäfer, H. Benz, W. Fiedler, A. Guggisberg, S. Bienz, M. Hesse, The Alkaloids 1994, 45, 1-125 | H. curta | HO 395 | Review | Link |
| Acylpolyamines: Mass spectrometric analytical methods for Araneidae spider acylpolyamines | Y. Itagaki, T. Nakajima, Toxin Rev. 2000, 19, 1, 23-52 | H. curta | HO 395 | Review | Link |
| The acylpolyamines from the venom of the spider Agelenopsis aperta | S. Chesnov, L. Bigler, M. Hesse, Helv. Chim. Acta 2001, 84, 2178-2197 | A. aperta | AG 395c | APCI-MS/MS | Link |
| Solid-phase synthesis of polyamine spider toxins and correlation with natural products by HPLC-MS/MS | N. Manov, M. Tzouros, S. Chesnov, L. Bigler, S. Bienz, Helv. Chim. Acta 2002, 85, 2827-2846 | A. aperta | | Synthesis, NMR, APCI-MS/MS | Link |
| Low molecular mass compounds in spider venom | Y. M. Forster, S. Bienz, L. Bigler, 2020, in preparation | div. | | | Link |
Spider species
| Spider species | Family | Discovered |
|---|
| Agelenopsis aperta | Agelenidae | 2001 / S. Chesnov |
| Agelenopsis potteri | Agelenidae | 2020 / Y. M. Forster |
| Hololena curta | Agelenidae | 1991 / G. B. Quistad |
| Hololena sp. | Agelenidae | 2020 / Y. M. Forster |