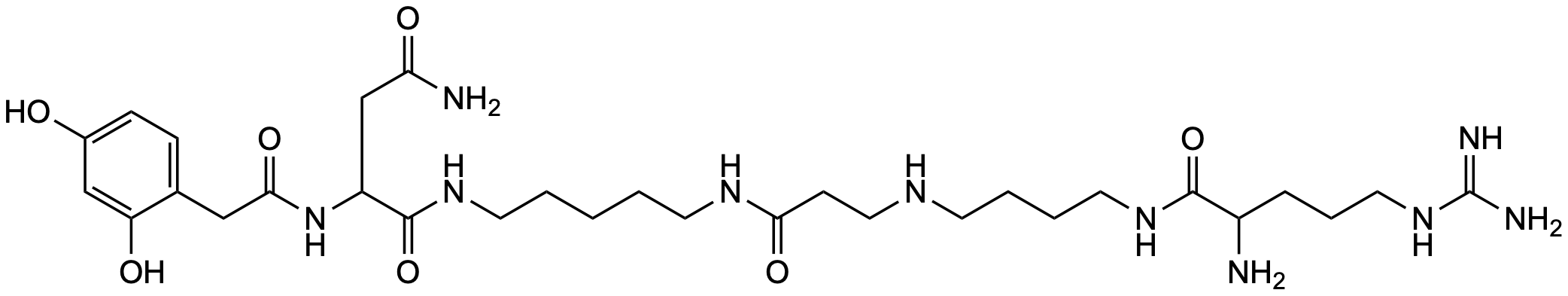
| Chemical characterization of spider toxin, JSTX and NSTX | Y. Aramaki, T. Yasuhara, T. Higashijima, M. Yoshioka, A. Miwa, N. Kawai, T. Nakajima, Proc. Jpn. Acad. 1986, 62, 359-362 | N. pilipes | NSTX 3 | NMR, Amino acid analysis | Link |
| Chemical characterization of spider toxin, NSTX | Y. Aramaki, T. Yasuhara, T. Higashijima, A. Miwa, N. Kawai, T. Nakajima, Biomed. Res. 1987, 8, 167 | N. pilipes | NSTX 3 | NMR, Amino acid analysis, Activity-studies | Link |
| Synthesis of a new neurotoxin NSTX-3 of Papua New Guinean spider | T. Teshima, T. Wakamiya, Y. Aramaki, T. Nakajima, N. Kawai, T. Shiba, Tetrahedron Letters 1987, 28, 3509-3510 | N. pilipes | NSTX 3 | Synthesis, NMR | Link |
| Synthesis of neurotoxic Nephila spider venoms: NSTX-3 and JSTX-3 | D. M. Nason, V. J. Jasys, P. R. Kelbaugh, D. Philips, N. A. Saccomano, R. A. Volkmann, Tetrahedron Letters 1989, 30, 2337-2340 | | NSTX 3 | Synthesis, NMR | Link |
| Characterization of binding sites for spider toxin, [3H]NSTX-3, in the rat brain | H. Ino, S. Nakade, M. Niinobe, K. Ikenaka, T. Teshima, T. Wakamiya, T. Matsumoto, T. Shiba, N. Kawai, K. Mikoshiba, Neuroscience Research 1990, 8, 29-39 | | NSTX 3 | Activity-studies | Link |
| Spider toxins , NSTX and JSTX block synaptic transmission in insect CNS | B. Hue, T. Piek, Comparative Biochemistry and Physiology, Part C: Pharmacology, Toxicology & Endocrinology 1990, 95C, 229-232 | | NSTX 3 | Activity-studies | Link |
| Structure-activity relationship of NSTX-3, spider toxin of Nephila maculata | T. Teshima, T. Matsumoto, T. Wakamiya, T. Shiba, T. Nakajima, N. Kawai, Tetrahedron 1990, 46, 3813-3818 | | NSTX 3 | Activity-studies | Link |
| Comparison of some arthropod toxins and toxin fragments as antagonists of excitatory amino acid-induced excitation of rat spinal neurons | M. G. Jones, D. Lodge, European Journal of Pharmacology 1991, 204, 203-209 | | NSTX 3 | Activity-studies | Link |
| Total synthesis of NSTX-3, spider toxin of Nephila maculata | T. Teshima, T. Matsumoto, T. Wakamiya, T. Shiba, Y. Aramaki, T. Nakajima, N. Kawai, Tetrahedron 1991, 47, 3305-3312 | | NSTX 3 (1) | Synthesis, NMR (ns), | Link |
| Antropod toxins as leads for novel insecticides: An assessment of polyamine amides as glutamate antagonists | I. S. Blagbrough, P. T. H. Brackley, M. Bruce, B. W. Bycroft, A. J. Mather, S. Millington, H. L. Sudan, P. N. R. Usherwood, Toxicon 1992, 30, 303-322 | | NSTX 3 | Review | Link |
| Polyamine toxins from spiders and wasps | A. Schäfer, H. Benz, W. Fiedler, A. Guggisberg, S. Bienz, M. Hesse, The Alkaloids 1994, 45, 1-125 | N. pilipes | NSTX 3 | Review | Link |
| Practical, convergent total synthesis of polyamine amide spider toxin NSTX-3 | I. S. Blagbrough, E. Moya, S. P. Walford, Tetrahedron Letters 1996, 37, 551-554 | | NSTX 3 (1) | Synthesis | Link |
| Diversity of Joro spider toxins | M. Yoshioka, 1997, 117, 700-714 | N. pilipes | NSTX 3 | Review | Link |
| Acylpolyamines: Mass spectrometric analytical methods for Araneidae spider acylpolyamines | Y. Itagaki , T. Nakajima , Toxin Rev. 2000, 19, 23-52 | N. pilipes | NSTX 3 | Review | Link |
| Novel mechanism of blocking axonal Na+ channels by three macrocyclic polyamine analogues and two spider toxins | M. Yakehiro, Y. Furukawa, T. Koike, E. Kimura, T. Nakajima, K. Yamaoka, I. Seyama, British Journal of Pharmacology 2001, 132, 63-72 | | NSTX 3 | Activity-studies | Link |
| An efficient and versatile synthesis of acylpolyamine spider toxins | K. Nihei, M. J. Kato, T. Yamane, M. S. Palma, K. Konno, Bioorg. Med. Chem. Lett. 2002, 12, 299-302 | | NSTX 3 | Synthesis | Link |
| A natural combinatorial chemistry strategy in acylpolyamine toxins from Nephilinae orb-web spiders | M. S. Palma, T. Nakajima, Toxin Rev. 2005, 24, 209-234 | N. borbonica & N. clavata | NSTX 3 | LC-MS | Link |
| Spider polyamine toxin | N. Kawai, Toxicon Review 2005, 24, 273-289 | | NSTX 3 | Review | Link |
| An efficient and versatile synthesis of all structural types of acylpolyamine spider toxins | K. Nihei, M. J. Kato, T. Yamane, K. Konno, Tetrahedron 2006, 62, 8335-8350 | | NSTX 3 (6) | Synthesis, NMR, ESI-MS/MS | Link |
| General synthesis of beta-alanine-containing spider polyamine toxins and discovery of Nephila polyamine toxins 1 and 8 as highly potent inhibitors of ionotropic glutamate receptors | S. Lucas, M. H. Poulsen, N. G. Norager, A. F. Barslund, T. B. Bach, A. S. Kristensen, K. Strømgaard, J. Med. Chem. 2012, 55, 10297-10301 | | NSTX 3 | Synthesis, NMR, Activity-studies | Link |
| Low molecular mass compounds in spider venom | Y. M. Forster, S. Bienz, L. Bigler, 2020, in preparation | div. | | | Link |