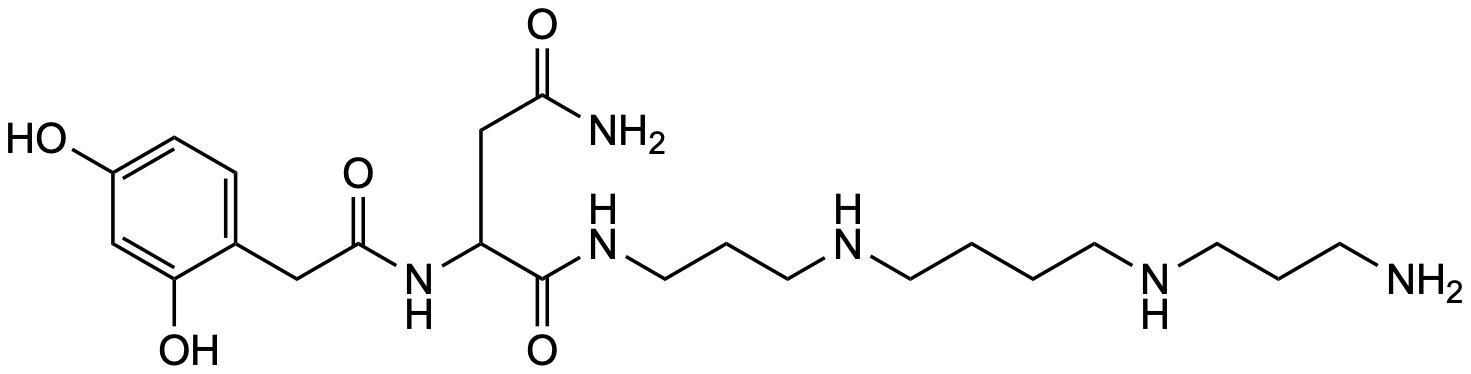
| Synthesis of spider toxin (JSTX-3) and its analogs | Y. Hashimoto, Y. Endo, K. Shudo, Y. Aramaki, N. Kawai, T. Nakajima, Tetrahedron Letters 1987, 28, 3511-3514 | | compound 4 | Synthesis, NMR, Activity-studies | Link |
| Newly synthesized analogues of the spider toxin block the crustacean glutamate receptor | K. Shudo, Y. Endo, Y. Hashimoto, Y. Aramaki, T. Nakajima, N. Kawai, Neuroscience Research 1987, 5, 82-85 | | C-2 | Synthesis, Activity-studies | Link |
| Use of spider toxin for the study of glutamate receptors | N. Kawai, T. Nakajima, Biomedical Research 1988, 9, 55-57 | | | ¹²⁵I-labeled toxin | |
| Acylpolyamines mimic the action of Joro spider toxin (JSTX) on crustacean muscle glutamate receptors | T. Asami, H. Kagechika, Y. Hashimoto, K. Shudo, A. Miwa, N. Kawai, T. Nakajima, Biomedical Research 1989, 10, 185-189 | | | Synthesis, NMR, Activity-studies | Link |
| Molecular structures of spider toxins (JSTX-1, 2, 3 and 4) in the venom of Nephila clavata L. Koch | T. Toki, T. Yasuhara, Y. Aramaki, Y. Hashimoto, K. Shudo, N. Kawai, T. Nakajima, Jpn. J. Sanit. Zool. 1990, 41, 9-14 | N. clavata | JSTX-1 | Amino acid analysis | Link |
| Purification of AMPA type glutamate receptor by a spider toxin | K. Shimazaki, H. P. C. Robinson, T. Nakajima, N. Kawai, T. Takenawa, Molecular Brain Research 1992, 13, 331-337 | | JSTX-1 | Activity-studies | Link |
| Polyamine toxins from spiders and wasps | A. Schäfer, H. Benz, W. Fiedler, A. Guggisberg, S. Bienz, M. Hesse, The Alkaloids 1994, 45, 1-125 | N. clavata | JSTX-1 | Review | Link |
| Structural characterization of glutaminergic blocker spider toxins by high-energy collision charge-remote fragmentations | T. Fujita, Y. Itagaki, H. Naoki, T. Nakajima, Rapid Commun. Mass Spectrom. 1995, 9, 365-371 | | JSTX-1 | CID of [M+Na]⁺ | Link |
| Diversity of Joro spider toxins | M. Yoshioka, 1997, 117, 700-714 | | JSTX-1 | Review | Link |
| Structural characterization of a new acylpolyaminetoxin from the venom of Brazilian garden spider Nephilenys cruentata | M. S. Palma, Y. Itagaki, T. Fujita, H. Naoki, T. Nakajima, Toxicon 1998, 36, 485-493 | N. cruentata | JSTX-1 | Frit-FAB-MS | Link |
| Development of a high-resolution MS-based method for the structural elucidation of polyamine spider toxins | S. Eichenberger, PhD-Thesis, University of Zurich 2009, 1-156 | L. patagiatus | LF 466D | nLC-ESI-MS/MS, Amino acid analysis | Link |
| Low molecular mass compounds in spider venom | Y. M. Forster, S. Bienz, L. Bigler, 2020, in preparation | div. | | | Link |